IDPD Delegation meets Chairman National Pharmaceutical Pricing Authority
Demands Rationalisation of prices of Drugs and Medical devices
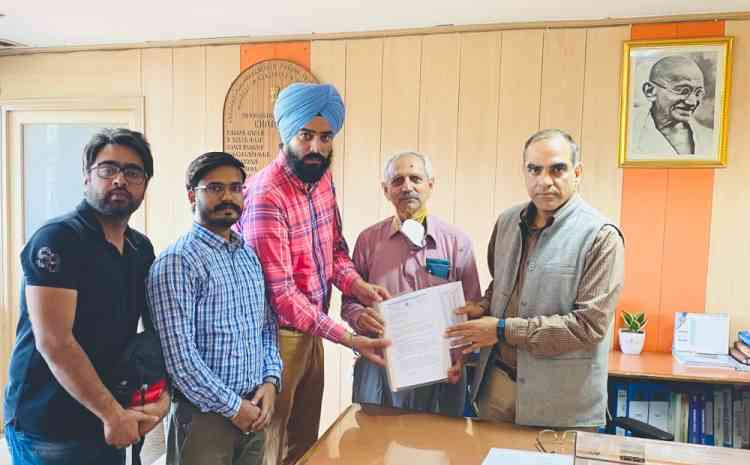
Ludhiana, October 14, 2022: A delegation of the Indian Doctors for Peace and Development (IDPD) led by Dr Arun Mitra, Senior Vice President IDPD, Dr Gurveer Singh, Dr Suraj Dhillon and Dr Seerat Sekhon met the chairman of the National Pharmaceutical Pricing Authority (NPPA) Kamlesh Kumar Pant and submitted memorandum demanding rationalisation of the prices of drugs and medical devices. They pointed out that the exorbitant prices of drugs because of high trade margins is adversely affecting the people even though the expenditure on drugs is around 67% of total out of pocket expenditure on health. In several cases there is exorbitant difference between the basic price of the drug and MRP in the case of generic (branded generics). This is totally unjustified and promotes malpractice. Similar is the price difference in some consumables/medical devices/ implants. The trade margin even in some branded drugs is glaring.
They pointed out that Committee on High Trade Margins in the Sale of Drugs submitted its report to the government on 9th December 2015, which found profit margin to be up to 5000% in some cases. After discussion with various stake holders, the committee gave recommendations to cap price of drugs but no action has been taken on that.
The delegation demanded:
1. The ex-factory price of the drugs should be calculated on the basis of cost
involved in its production.
2. Cap the trade margin to a maximum of 30% from the factory price to the consumer. Opt for alternate formula - Landed cost/ ex- factory cost + 30% trade margin = MRP, inclusive of retail and wholesale margin, logistics, inventory cost.
3. All medicines must bear the cost price and MRP so as to make the profit
margin transparent.
4. All the chemicals which are labelled as medicines should be included in the National List of Essential Medicines (NLEM), because medicines are not a choice of the patient.
5. All the branded medicines should be sold with the chemical/pharmacological
name only. The name of the company be mentioned on the cover separately. There should be no trade names.
6. Presently there is huge difference between the basic price and the MRP in case of the generic (Branded Generic) drugs. This should be made reasonable to prevent exploitation of the patient.
It is worthwhile to mention that after several rounds of meetings with the NPPA in 2016, 2017 & 2020 the prices coronary stents were curtailed by the NPPA.
The chairman assured of serious consideration to the demands and told that soon they will come out with a report on Trade Margin Rationalisation on several drugs. The IDPD delegation told him that they would meet the Health Ministry regarding the National List of Essential Medicines and also the National Medical Commission for related issues. The Chairman in a happy note asked the delegation to keep him apprised in this regard in future as well.
The IDPD will generate public opinion on the issues of drug prices through seminars, lectures and media interactions.


 City Air News
City Air News 










