UK begins first human trial of new Zika vaccine
The UK has begun the first human clinical trials of a new Zika vaccine, after it showed promising results in animal studies.
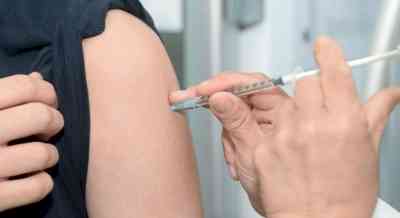
London, April 29 (IANS) The UK has begun the first human clinical trials of a new Zika vaccine, after it showed promising results in animal studies.
The vaccine is being trialled by the University of Liverpool.
India is also reportedly developing a vaccine against Zika.
Although now not as prevalent as during its peak in 2016, Zika remains an ongoing threat, with thousands of cases of the mosquito-borne virus reported each year, mainly in countries close to the equator.
Pregnant women continue to be the population at highest risk for the infection as the virus can cause severe foetal birth defects.
It's hoped that the vaccine, designed to be suitable for use during pregnancy, will generate highly protective and long-lasting immunity.
Having shown promising results in animal studies, the vaccine has now moved into a 'first in human' Phase I trial.
If successful, the new trial could lead to a major breakthrough in tackling the Zika virus, for which there are still no approved vaccines or treatments available anywhere in the world.
"Zika should not be forgotten especially since climate change is contributing to the spread of the Aedes mosquitoes (the mosquitoes that can carry the Zika virus) to countries where immunity is not there. Vaccines like ours will enable us to be better prepared for the next Zika outbreak," said Dr Krishanthi Subramaniam, a tenure-track research fellow who led on the studies demonstrating the effectiveness of the vaccine to lower virus levels in animals.
Liverpool researchers used an approach to develop a vaccine based on studies to understand immunity to Zika and other related viruses.
The trial is open to healthy individuals aged between 18-59.
Healthy volunteers recruited to the trial will receive two doses of the new vaccine to evaluate its safety, tolerability and its ability to produce an immune response.
The vaccine will be assessed in groups of four volunteers at a time, with numbers increasing as evidence of safety accumulates.
Up to 40 volunteers in this phase of work is planned which will be taking place over the next nine months.
In addition, the performance of the vaccine will also be assessed in people who have had exposure to other viruses that circulate in the places where Zika virus is found, such as dengue virus, or yellow fever vaccine.
The vaccine work was supported by a 4.7 million pounds Innovate UK SBRI Vaccines for Global Epidemics award and includes collaborators from the University of Manchester, the UK Health Security Agency and industry.


 IANS
IANS 










