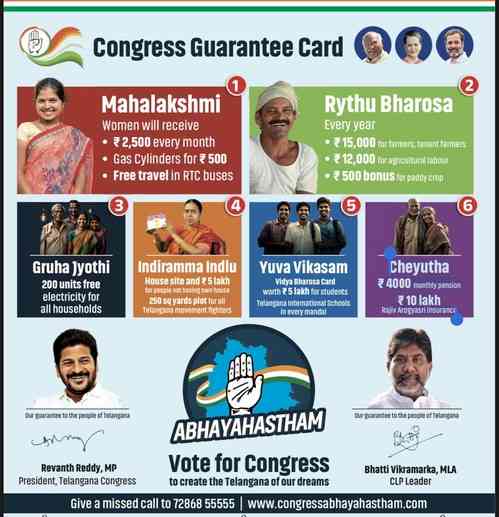
Call for separate regulations for medical devices and OTC drugs
Author(s): City Air NewsMr Dues Mubangizi, Dr Mahur Gupta, Dr Gaby Vercauteren, Dr Manisha Shridhar and Mr Emmanuel Patras in a joint session, at the Medical Devices and Indian Pharma Conference, 2019. 4th International Conference on Pharma...


Mr Dues Mubangizi, Dr Mahur Gupta, Dr Gaby Vercauteren, Dr Manisha Shridhar and Mr Emmanuel Patras in a joint session, at the Medical Devices and Indian Pharma Conference, 2019.
4th International Conference on Pharma & Medical Devices concludes
Chennai, February 21, 2019: The 4th International Conference on Pharmaceuticals and Medical Devices which concluded witnessed the participation of drug regulators from over 30 countries including Russia, Kenya, the UK, Malaysia, Indonesia, Saudi Arabia and Uzbekistan, along with participation of Indian drug regulators from 15 states.
The conference was organised by the Department of Pharmaceuticals, Ministry of Chemicals & Fertilizers and Government of India jointly with the Federation of Indian Chambers of Commerce & Industry (FICCI).
Some of the issues discussed at the CEOs roundtable included development of separate medical device and over-the-counter (OTC) drug regulations act, early decision on implementation of trade margin rationalization, addressing the issue of Active Pharmaceutical Ingredients (APIs) price escalation imported from China, incentivisation for innovation in the pharmaceutical and medical device sector, greater transparency in the functioning of National Pharmaceutical Pricing Authority (NPPA) and last but not the least, amendments by the Ministry of Consumer Affairs for allowing companies to utilise the mandatory CSR spending towards medicine donations into Jan Aushadhi or Ayushman Bharat scheme.
Knowledge sessions were focused towards promoting innovative ecosystem in India for pharmaceutical industry, interaction with State Drug Controllers and CDSCO for facilitating ease of doing business, initiatives for import registration procedure to enable faster access to global market for the industry, WHO activities and plans related to the regulation of health technologies and enabling the industry to gear up for Ayushman Bharat, the most aspirational health scheme rolled out by the government.
In his concluding remarks, Mr. Navdeep Rinwa, Joint Secretary, Department of Pharmaceutical, Government of India, mentioned how the government was prioritising the pharma and medical device sector in AI applications by bringing in task forces and other policies in place.

 cityairnews
cityairnews